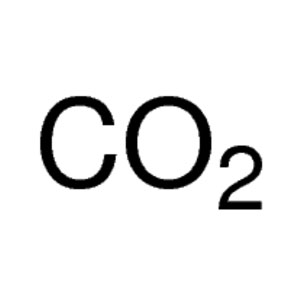Dry ice, sometimes referred to as "cardice" (chiefly by British chemists), is the solid form of carbon dioxide. It is used primarily as a cooling agent. Its advantages include lower temperature than that of water ice and not leaving any residue (other than incidental frost from moisture in the atmosphere). It is useful for preserving frozen foods where mechanical cooling is unavailable.
Dry ice sublimates at 194.65 K (−78.5 °C; −109.3 °F), at Earth atmospheric pressures. This extreme cold makes the solid dangerous to handle without protection due to burns caused by freezing (frostbite). While generally not very toxic, the outgassing from it can cause hypercapnia (abnormally elevated carbon dioxide levels in the blood) due to buildup in confined locations.
Properties
Dry ice is the solid form of carbon dioxide (CO2), a molecule consisting of a single carbon atom bonded to two oxygen atoms. Dry ice is colorless, non-flammable, with a sour zesty odor, and can lower the pH of a solution when dissolved in water, forming carbonic acid (H2CO3).
The density of dry ice varies, but usually ranges between about 1.4 and 1.6 g/cm3 (87 and 100 lb/cu ft). The low temperature and direct sublimation to a gas makes dry ice an effective coolant, since it is colder than water ice and leaves no residue as it changes state. Its enthalpy of sublimation is 571 kJ/kg (25.2 kJ/mol).At pressures below 5.13 atm and temperatures below −56.4 °C (−69.5 °F) (the triple point), CO2 changes from a solid to a gas with no intervening liquid form, through a process called sublimation.[note 1] The opposite process is called deposition, where CO2 changes from the gas to solid phase (dry ice). At atmospheric pressure, sublimation/deposition occurs at −78.5 °C (−109.3 °F) or 194.65 K.
Dry ice is non-polar, with a dipole moment of zero, so attractive intermolecular van der Waals forces operate. The composition results in low thermal and electrical conductivity.
Applications
Commercial
The most common use of dry ice is to preserve food, using non-cyclic refrigeration.
It is frequently used to package items that must remain cold or frozen, such as ice cream or biological samples, without the use of mechanical cooling.
Dry ice can be used to flash-freeze food or laboratory biological samples, carbonate beverages, make ice cream, solidify oil spills and stop ice sculpturesand ice walls from melting.
Dry ice can be used to arrest and prevent insect activity in closed containers of grains and grain products, as it displaces oxygen, but does not alter the taste or quality of foods. For the same reason, it can prevent or retard food oils and fats from becoming rancid.
When dry ice is placed in water, sublimation is accelerated, and low-sinking, dense clouds of smoke-like fog are created. This is used in fog machines, at theaters, haunted house attractions, and nightclubs for dramatic effects. Unlike most artificial fog machines, in which fog rises like smoke, fog from dry ice hovers near the ground. Dry ice is useful in theater productions that require dense fog effects. The fog originates from the bulk water into which the dry ice is placed, and not from atmospheric water vapor (as is commonly assumed).
It is occasionally used to freeze and remove warts. However, liquid nitrogen performs better in this role, since it is colder so requires less time to act, and less pressure. Dry ice has fewer problems with storage, since it can be generated from compressed carbon dioxide gas as needed.
Plumbers use equipment that forces pressurised liquid CO2 into a jacket around a pipe. The dry ice formed causes the water to freeze, forming an ice plug, allowing them to perform repairs without turning off the water mains. This technique can be used on pipes up to 4 inches (100 mm) in diameter.
Dry ice can be used as bait to trap mosquitoes, bedbugs, and other insects, due to their attraction to carbon dioxide.
It can be used to exterminate rodents. This is done by dropping pellets into rodent tunnels in the ground and then sealing off the entrance, thus suffocating the animals as the dry ice sublimates.
Tiny dry ice pellets can be used to fight fire by both cooling fuel and suffocating the fire by excluding oxygen.
The extreme temperature of dry ice can cause viscoelastic materials to change to glass phase. Thus it is useful for removing many types of pressure sensitive adhesives.
Industrial
Dry ice can be used for loosening asphalt floor tiles or car sound deadening material making it easy to prise off, as well as freezing water in valveless pipes to enable repair.
One of the largest mechanical uses of dry ice is blast cleaning. Dry ice pellets are shot from a nozzle with compressed air, combining the power of the speed of the pellets with the action of the sublimation. This can remove residues from industrial equipment. Examples of materials removed include ink, glue, oil, paint, mold and rubber. Dry ice blasting can replace sandblasting, steam blasting, water blasting or solvent blasting. The primary environmental residue of dry ice blasting is the sublimed CO2, thus making it a useful technique where residues from other blasting techniques are undesirable. Recently, blast cleaning has been introduced as a method of removing smoke damage from structures after fires.
Dry ice is also useful for the de-gassing of flammable vapours from storage tanks — the sublimation of dry ice pellets inside an emptied and vented tank causes an outrush of CO2 that carries with it the flammable vapours.
The removal and fitting of cylinder liners in large engines requires the use of dry ice to chill and thus shrink the liner so that it freely slides into the engine block. When the liner then warms up, it expands, and the resulting interference fit holds it tightly in place. Similar procedures may be used in fabricating mechanical assemblies with a high resultant strength, replacing the need for pins, keys or welds.
Dry-ice blasting, a form of carbon dioxide cleaning, is used in a number of industrial applications.
It is also useful as a cutting fluid.
Scientific
In laboratories, a slurry of dry ice in an organic solvent is a useful freezing mixture for cold chemical reactions and for condensing solvents in rotary evaporators. Dry ice/acetone forms a cold bath of −78 °C, which can be used for instance to prevent thermal runaway in a Swern oxidation.
The process of altering cloud precipitation can be done with the use of dry ice. It was widely used in experiments in the US in the 1950s and early 60s before it was replaced by silver iodide. Dry ice has the advantage of being relatively cheap and completely non-toxic. Its main drawback is the need to be delivered directly into the supercooled region of clouds being seeded.
Dry Ice Applications
There are a wide variety of dry ice applications. Below we list some of the common consumer and domestic uses of dry ice, along with suggested products for each application.
Chilled and Frozen Storage
Maintaining produce at chilled temperatures
If you want to keep produce at chilled temperatures, then use smaller amounts of dry ice in the container and don’t place it directly on the food or drink, but rather in a side compartment or with some kind of buffer or packing between the dry ice and the food. Gel packs are ideal if you only want to keep food and drink chilled without any risk of freezing. Soak Gel packs in water and then place in your freezer to prepare them. You can then distribute them evenly throughout your cooler.
Maintaining produce at frozen temperatures
If you want to keep your produce frozen then use dry ice. Dry Ice slices are ideal as they are individually wrapped and easy to distribute across your cooler or storage carton. 10kgs of dry ice would be suitable for 48 hours of storage. Remember, the better insulated the container the longer the dry ice will last. Fill any remaining airspace in the container with newspaper or some kind of packaging. This helps to reduce the rate at which the dry ice sublimates (turns from a solid to a gas form).
Airline Shipping
Dry ice is widely used for airline shipping and is identified by a Class 9 UN1845 label. We supply these labels on our online store. For assistance calculating how much dry ice you will require, please contact our team with the internal dimensions of your storage container and the expected shipping time. As ambient temperatures can impact the longevity of the dry ice, we recommend using slightly more dry ice than you expect to need or using a Thermosafe Insulated Shipper. These are available in two different sizes and because they offer a greater level of insulation they will hold the dry ice for a longer period of time.
Special Effects
Weddings – First Dance
Create a romantic dancing-on-clouds effect for your first dance. Dry ice produces the perfect low-lying fog that flows across the ground. A dedicated dry ice smoke machine should be used with the dry ice. The recommended type of dry ice is dependent on the type of dry ice smoke machine that you hire. Typically dry ice pellets or a 10kg block of dry ice is best. The hire company will be able to advise you which is most suitable.
Champagne Reception
Dry ice is a spectacular addition to a champagne glass tower, creating a cascading fog effect. Always use ChilliSticks when adding dry ice to any drinks intended for consumption. ChilliSticks are specially developed to hold dry ice pellets safely in a glass without risk of swallowing the dry ice. Dry Ice should never be added loose into drinks – the extremely low temperature of the dry ice (-78oC) could result in internal injuries if consumed.
Halloween Special Effects
Dry ice can create a wide variety of fun Halloween themed effects, from cauldrons of bubbling fog to eerie graveyards! We love to see our customers creations each year and you can see some Pellets are the most popular form of dry ice at Halloween time as they are easy to use in small quantities and to create a number of different effects. Hosting a Halloween party? You can purchase different size party packs from our online store that include dry ice, chill sticks, insulated gloves and a dry ice scoop.
Food and Drink Presentation
Restaurants – Food Presentation
Dry ice is sometimes used in the presentation of food in restaurants. Dry Ice pellets work best as you can add small amounts to serving trays to produce a fog effect around the food dishes. Add the dry ice pellets to warm water to produce the desired fog vapour.
Bars – Dry Ice Cocktails
Add Dry ice to cocktails and other drinks to produce a fun and unique fog effect. ChilliSticks are specially developed to hold dry ice pellets safely in a glass without risk of the dry ice being swallowed. You can preload them with dry ice pellets and then submerge the ChilliSticks in the remaining dry ice ready for use. Never add Dry Ice loose into drinks – the extremely low temperature of the dry ice (-78oC) could results in internal injuries if swallowed.
Homemade ice-cream
You can use dry ice to make homemade ice cream. Crush dry ice pellets and add them to the ice-cream ingredients until the mixture reaches the desired consistency. Carbon dioxide bubbles can become trapped in the mixture, resulting in a carbonated ice-cream.
How to Make Home Made ICE CREAM Less than a Minute
Click Below Link ... Click
Agriculture
Freezebranding
With a temperature of -78oC, Dry Ice blocks or slices are most suitable for freeze-branding. Place the dry ice into a container and pour methylated spirits over the Dry Ice. Place the branding iron into this super-cooled alcohol mixture. Once sufficiently cooled, press it against the animal’s hide. This applys the permanent brand mark without damaging the hide.